Gas
From Unofficial Stationeers Wiki
Revision as of 20:30, 13 April 2024 by Fridfinnurm (talk | contribs) (Added a combined phase change diagram.)
Revision as of 20:30, 13 April 2024 by Fridfinnurm (talk | contribs) (Added a combined phase change diagram.)
This is a disambiguation page, which lists pages which may be the intended target. If a page link referred you here, please consider editing it to point directly to the intended page.
Gas may refer to:
- Atmosphere
- Carbon Dioxide (CO2)
- Fuel (66.6% H2 + 33.3% O2)
- Hydrogen (H2)
- Nitrogen (N2)
- Nitrous Oxide (N2O)
- Oxygen (O2)
- Pollutants (X)
- Volatiles
- Water (H2O)
Gases
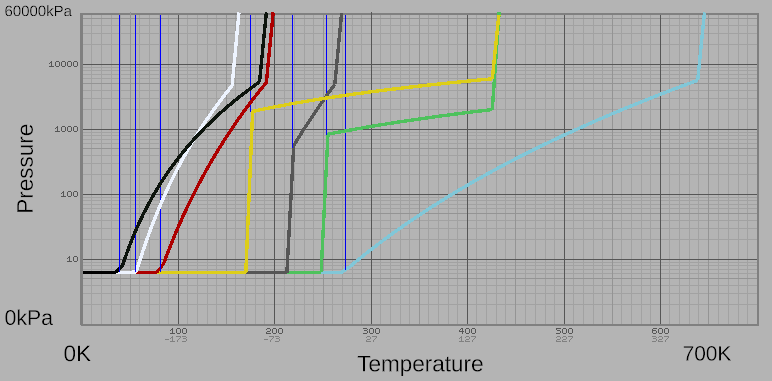
Stationpedia has simple phase diagrams for all Gases, shown in composite below. A more complex phase change diagram can be located Here
| Name | Filter Color | Molar Heat Capacity (J⋅K−1⋅mol−1) |
Molar Heat of Fusion (kJ⋅K−1⋅mol−1) |
Melting Point (K / °C) |
Boiling Point at 100 kPa (K / °C) |
Minimum Condensation (kPa at K / °C) |
Maximum Liquid Temperature (K / °C at kPa) |
|---|---|---|---|---|---|---|---|
| Carbon Dioxide (CO2) | Gray | 28.2 | 0.6 | 218 / -55.3 | N/A | 517 at 218 / -55.3 | 265 / -8.1 at 6000 |
| Nitrogen (N2) | Black | 20.6 | 0.5 | 40 / -233 | 75 / -198 | 6.3 at 40 / -233 | 190 / -83 at 6000 |
| Nitrous Oxide (N2O) | Green | 37.2 | 4 | 252 / -21 | N/A | 800 at 252 / -21 | 431 / 158 at 2000 |
| Oxygen (O2) | White | 21.1 | 0.8 | 56.4 / -217 | 90 / -183 | 6.3 at 56.4 / -217 | 162 / -111 at 6000 |
| Pollutant (X) | Yellow | 24.8 | 2 | 173 / -99.8 | N/A | 1800 at 173 / -99.8 | 425 / 152 at 6000 |
| Volatiles (H2) | Red | 20.4 | 1 | 81.6 / -192 | 112 / -162 | 6.3 at 81.6 / -192 | 195 / -78.1 at 6000 |
| Water (H20) | Blue | 72 | 8 | 273 / 0 | 373 / 100 | 6.3 at 273 / 0 | 643 / 370 at 6000 |
Phase changes have been added to the game system and most gases now have an associated Liquid and a pure solid phase.
The following exist only in solid state and have no gaseous form: Charcoal, Coal, Silicon, Iron, Copper, Nickel, Silver, Gold, Lead, Uranium.
Mixtures
| Name | Contents | Tank Color | Notes |
|---|---|---|---|
| Fuel | 67% H2, 33% O2 | Orange | Autoignition at 573.15 K / 300 °C |
| HydroNox | 50% H2, 50% N2O | N/A | Autoignition at 323.15 K / 50 °C |
| Air | 75% N2, 25% O2 | White | 16 kPa Partial Pressure O2 minimum is required for human player breathing. Hyperoxia is not simulated.(NOTE: Suit air mixture should be 100% O2) |
Simulation Mechanics
| Concept | Simulated | Notes |
|---|---|---|
| Ideal Gas Law (PV=nRT) | Partial | Pressure will change with temperature, volume, and moles; Temperature will not change |
| Thermal Conductivity | No | Rate of energy transfer is the same for all substances |
| Thermal Radiation | Partial | Radiated heat is not absorbed by nearby objects |
| Phase Change | Partial | Gas/Liquid have simplified graph; Solid has a fixed temperature; Plasma is ignored |
| Fluid Dynamics | No | 'Convection' parts work in pressurized environment, other parts work in vacuum |
| Gas Stoichiometry | No | The various reactions are arbitrary and vary depending on device used for different products from the same reactants |
| Gravity/Density | No | Gas/Liquid is present in all directions |
| Environment Volume | Yes | Each environment cube acts as a single point with fixed volume |
| Pipe Volume | Yes | Each pipe network acts as a single point with fixed volume depending on number of pipes segments |
| Flow Direction | Yes | All pumps and mixer act as check valve, passive check valve available, other valves are not one-way |
| Pipe Integrity | Yes | Liquid pipes hold low pressure, but may contain both liquid and gas without consequence; Gas pipes hold high pressure, but stress and break with too much liquid |
| Heat Transfer | Partial | Liquid temperature is controlled by gas temperature (if no gas, liquid heater will not function) |