Difference between revisions of "Gas"
From Unofficial Stationeers Wiki
(→Gases: Added molar mass) |
|||
| (7 intermediate revisions by 5 users not shown) | |||
| Line 17: | Line 17: | ||
==Gases== | ==Gases== | ||
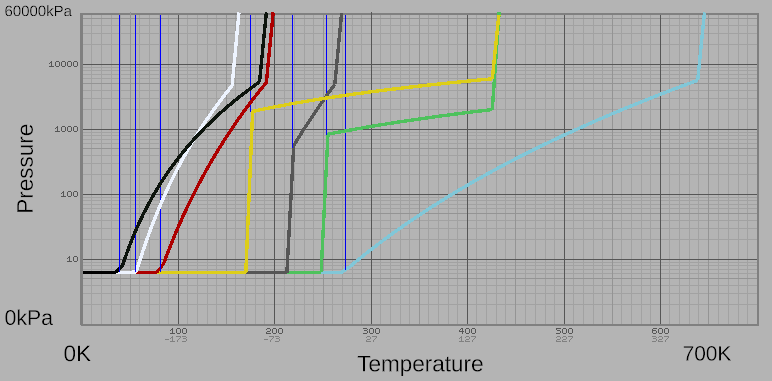
| − | Stationpedia has simple phase diagrams for all Gases, | + | Stationpedia has simple phase diagrams for all Gases, shown in composite below. A more complex phase change diagram can be located [https://www.desmos.com/calculator/voj4scjipd Here] |
| + | |||
| + | [[File:Combined phase change diagram.png|frame|none|''A combined phase change diagram showing the phase changes of all in-game gasses.'']] | ||
| + | |||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
| − | ! Name !! Filter Color !! Molar Heat Capacity<br>(J⋅K<sup>−1</sup>⋅mol<sup>−1</sup>) !! Molar Heat of Fusion<br>(kJ⋅K<sup>−1</sup>⋅mol<sup>−1</sup>) !! Melting Point<br>(K / °C) !! Boiling Point at 100 kPa<br>(K / °C) !! Minimum Condensation<br>(kPa at K / °C) !! Maximum Liquid Temperature<br>(K / °C at kPa) | + | ! Name !! Filter Color !! Molar Heat Capacity<br>(J⋅K<sup>−1</sup>⋅mol<sup>−1</sup>) !! Molar Heat of Fusion<br>(kJ⋅K<sup>−1</sup>⋅mol<sup>−1</sup>) !! data-sort-type="number" | Melting Point<br>(K / °C) !! data-sort-type="number" | Boiling Point at 100 kPa<br>(K / °C) !! data-sort-type="number" | Minimum Condensation<br>(kPa at K / °C) !! data-sort-type="number" | Maximum Liquid Temperature<br>(K / °C at kPa) !! Liquid Molar Volume<br>(L / mol) !! Molar Mass<br>(g / mol) |
|- | |- | ||
| − | | [[Carbon Dioxide]] (CO<sub>2</sub>) || Gray || 28.2 || 0.6 || 218 / -55.3 || N/A || 517 at 218 / -55.3 || 265 / -8.1 at 6000 | + | | [[Carbon Dioxide]] (CO<sub>2</sub>) || Gray || 28.2 || 0.6 || 218 / -55.3 || N/A || 517 at 218 / -55.3 || 265 / -8.1 at 6000 || 0.04 || 44 |
|- | |- | ||
| − | | [[Nitrogen]] (N<sub>2</sub>) || Black || 20.6 || 0.5 || 40 / -233 || 75 / -198 || 6.3 at 40 / -233 || 190 / -83 at 6000 | + | | [[Nitrogen]] (N<sub>2</sub>) || Black || 20.6 || 0.5 || 40 / -233 || 75 / -198 || 6.3 at 40 / -233 || 190 / -83 at 6000 || 0.0348 || 64 |
|- | |- | ||
| − | | [[Nitrous Oxide]] (N<sub>2</sub>O) || Green || 37.2 || 4 || 252 / -21 || N/A || 800 at 252 / -21 || 431 / 158 at 2000 | + | | [[Nitrous Oxide]] (N<sub>2</sub>O) || Green || 37.2 || 4 || 252 / -21 || N/A || 800 at 252 / -21 || 431 / 158 at 2000 || 0.026 || 46 |
|- | |- | ||
| − | | [[Oxygen]] (O<sub>2</sub>) || White || 21.1 || 0.8 || 56.4 / -217 || 90 / -183 || 6.3 at 56.4 / -217 || 162 / -111 at 6000 | + | | [[Oxygen]] (O<sub>2</sub>) || White || 21.1 || 0.8 || 56.4 / -217 || 90 / -183 || 6.3 at 56.4 / -217 || 162 / -111 at 6000 || 0.03 || 16 |
|- | |- | ||
| − | | [[Pollutant]] (X) || Yellow || 24.8 || 2 || 173 / -99.8 || N/A || 1800 at 173 / -99.8 || 425 / 152 at 6000 | + | | [[Pollutant]] (X) || Yellow || 24.8 || 2 || 173 / -99.8 || N/A || 1800 at 173 / -99.8 || 425 / 152 at 6000 || 0.04 || 28 |
|- | |- | ||
| − | | [[Volatiles]] (H<sub>2</sub>) || Red || 20.4 || 1 || 81.6 / -192 || 112 / -162 || 6.3 at 81.6 / -192 || 195 / -78.1 at 6000 | + | | [[Volatiles]] (H<sub>2</sub>) || Red || 20.4 || 1 || 81.6 / -192 || 112 / -162 || 6.3 at 81.6 / -192 || 195 / -78.1 at 6000 || 0.04 || 16 |
|- | |- | ||
| − | | [[Water]] (H<sub>2</sub>0) || Blue || 72 || 8 || 273 / 0 || 373 / 100 || 6.3 at 273 / 0 || 643 / 370 at 6000 | + | | [[Water]] (H<sub>2</sub>0) || Blue || 72 || 8 || 273 / 0 || 373 / 100 || 6.3 at 273 / 0 || 643 / 370 at 6000 || 0.018 || 18 |
|} | |} | ||
| Line 51: | Line 54: | ||
| [[HydroNox]] || 50% H<sub>2</sub>, 50% N<sub>2</sub>O || N/A || Autoignition at 323.15 K / 50 °C | | [[HydroNox]] || 50% H<sub>2</sub>, 50% N<sub>2</sub>O || N/A || Autoignition at 323.15 K / 50 °C | ||
|- | |- | ||
| − | | [[Air]] || 75% N<sub>2</sub>, 25% O<sub>2</sub> || White || 16 kPa Partial Pressure O<sub>2</sub> minimum is required for human player breathing. Hyperoxia is not simulated. | + | | [[Air]] || 75% N<sub>2</sub>, 25% O<sub>2</sub> || White || 16 kPa Partial Pressure O<sub>2</sub> minimum is required for human player breathing. Hyperoxia is not simulated.(NOTE: Suit air mixture should be 100% O<sub>2</sub>) |
|} | |} | ||
==Simulation Mechanics== | ==Simulation Mechanics== | ||
| − | + | {| class="wikitable" | |
| − | + | |- | |
| − | + | ! Concept | |
| − | + | ! Simulated | |
| − | + | ! Notes | |
| − | + | |- | |
| − | + | | Ideal Gas Law (PV=nRT) | |
| − | + | | Partial | |
| − | + | | Pressure will change with temperature, volume, and moles; Temperature will not change | |
| − | + | |- | |
| − | + | | Thermal Conductivity | |
| − | + | | No | |
| + | | Rate of energy transfer is the same for all substances | ||
| + | |- | ||
| + | | Thermal Radiation | ||
| + | | Partial | ||
| + | | Radiated heat is not absorbed by nearby objects | ||
| + | |- | ||
| + | | Phase Change | ||
| + | | Partial | ||
| + | | Gas/Liquid have simplified graph; Solid has a fixed temperature; Plasma is ignored | ||
| + | |- | ||
| + | | Fluid Dynamics | ||
| + | | No | ||
| + | | 'Convection' parts work in pressurized environment, other parts work in vacuum | ||
| + | |- | ||
| + | | Gas Stoichiometry | ||
| + | | No | ||
| + | | The various reactions are arbitrary and vary depending on device used for different products from the same reactants | ||
| + | |- | ||
| + | | Gravity/Density | ||
| + | | No | ||
| + | | Gas/Liquid is present in all directions | ||
| + | |- | ||
| + | | Environment Volume | ||
| + | | Yes | ||
| + | | Each environment cube acts as a single point with fixed volume | ||
| + | |- | ||
| + | | Pipe Volume | ||
| + | | Yes | ||
| + | | Each pipe network acts as a single point with fixed volume depending on number of pipes segments | ||
| + | |- | ||
| + | | Flow Direction | ||
| + | | Yes | ||
| + | | All pumps and mixer act as check valve, passive check valve available, other valves are not one-way | ||
| + | |- | ||
| + | | Pipe Integrity | ||
| + | | Yes | ||
| + | | Liquid pipes hold low pressure, but may contain both liquid and gas without consequence; Gas pipes hold high pressure, but stress and break with too much liquid | ||
| + | |- | ||
| + | | Heat Transfer | ||
| + | | Partial | ||
| + | | Liquid temperature is controlled by gas temperature (if no gas, liquid heater will not function) | ||
| + | |} | ||
[[Category:Gas]][[Category:Liquid]] | [[Category:Gas]][[Category:Liquid]] | ||
Latest revision as of 19:06, 25 August 2024
This is a disambiguation page, which lists pages which may be the intended target. If a page link referred you here, please consider editing it to point directly to the intended page.
Gas may refer to:
- Atmosphere
- Carbon Dioxide (CO2)
- Fuel (66.6% H2 + 33.3% O2)
- Hydrogen (H2)
- Nitrogen (N2)
- Nitrous Oxide (N2O)
- Oxygen (O2)
- Pollutants (X)
- Volatiles
- Water (H2O)
Gases[edit]
Stationpedia has simple phase diagrams for all Gases, shown in composite below. A more complex phase change diagram can be located Here
| Name | Filter Color | Molar Heat Capacity (J⋅K−1⋅mol−1) |
Molar Heat of Fusion (kJ⋅K−1⋅mol−1) |
Melting Point (K / °C) |
Boiling Point at 100 kPa (K / °C) |
Minimum Condensation (kPa at K / °C) |
Maximum Liquid Temperature (K / °C at kPa) |
Liquid Molar Volume (L / mol) |
Molar Mass (g / mol) |
|---|---|---|---|---|---|---|---|---|---|
| Carbon Dioxide (CO2) | Gray | 28.2 | 0.6 | 218 / -55.3 | N/A | 517 at 218 / -55.3 | 265 / -8.1 at 6000 | 0.04 | 44 |
| Nitrogen (N2) | Black | 20.6 | 0.5 | 40 / -233 | 75 / -198 | 6.3 at 40 / -233 | 190 / -83 at 6000 | 0.0348 | 64 |
| Nitrous Oxide (N2O) | Green | 37.2 | 4 | 252 / -21 | N/A | 800 at 252 / -21 | 431 / 158 at 2000 | 0.026 | 46 |
| Oxygen (O2) | White | 21.1 | 0.8 | 56.4 / -217 | 90 / -183 | 6.3 at 56.4 / -217 | 162 / -111 at 6000 | 0.03 | 16 |
| Pollutant (X) | Yellow | 24.8 | 2 | 173 / -99.8 | N/A | 1800 at 173 / -99.8 | 425 / 152 at 6000 | 0.04 | 28 |
| Volatiles (H2) | Red | 20.4 | 1 | 81.6 / -192 | 112 / -162 | 6.3 at 81.6 / -192 | 195 / -78.1 at 6000 | 0.04 | 16 |
| Water (H20) | Blue | 72 | 8 | 273 / 0 | 373 / 100 | 6.3 at 273 / 0 | 643 / 370 at 6000 | 0.018 | 18 |
Phase changes have been added to the game system and most gases now have an associated Liquid and a pure solid phase.
The following exist only in solid state and have no gaseous form: Charcoal, Coal, Silicon, Iron, Copper, Nickel, Silver, Gold, Lead, Uranium.
Mixtures[edit]
| Name | Contents | Tank Color | Notes |
|---|---|---|---|
| Fuel | 67% H2, 33% O2 | Orange | Autoignition at 573.15 K / 300 °C |
| HydroNox | 50% H2, 50% N2O | N/A | Autoignition at 323.15 K / 50 °C |
| Air | 75% N2, 25% O2 | White | 16 kPa Partial Pressure O2 minimum is required for human player breathing. Hyperoxia is not simulated.(NOTE: Suit air mixture should be 100% O2) |
Simulation Mechanics[edit]
| Concept | Simulated | Notes |
|---|---|---|
| Ideal Gas Law (PV=nRT) | Partial | Pressure will change with temperature, volume, and moles; Temperature will not change |
| Thermal Conductivity | No | Rate of energy transfer is the same for all substances |
| Thermal Radiation | Partial | Radiated heat is not absorbed by nearby objects |
| Phase Change | Partial | Gas/Liquid have simplified graph; Solid has a fixed temperature; Plasma is ignored |
| Fluid Dynamics | No | 'Convection' parts work in pressurized environment, other parts work in vacuum |
| Gas Stoichiometry | No | The various reactions are arbitrary and vary depending on device used for different products from the same reactants |
| Gravity/Density | No | Gas/Liquid is present in all directions |
| Environment Volume | Yes | Each environment cube acts as a single point with fixed volume |
| Pipe Volume | Yes | Each pipe network acts as a single point with fixed volume depending on number of pipes segments |
| Flow Direction | Yes | All pumps and mixer act as check valve, passive check valve available, other valves are not one-way |
| Pipe Integrity | Yes | Liquid pipes hold low pressure, but may contain both liquid and gas without consequence; Gas pipes hold high pressure, but stress and break with too much liquid |
| Heat Transfer | Partial | Liquid temperature is controlled by gas temperature (if no gas, liquid heater will not function) |