Difference between revisions of "Gas"
From Unofficial Stationeers Wiki
(→Mixtures) |
|||
| (30 intermediate revisions by 15 users not shown) | |||
| Line 2: | Line 2: | ||
<translate> | <translate> | ||
{{Disambiguation}} | {{Disambiguation}} | ||
| − | + | In older versions of Stationeers, Gas was a generalized term for fluid substances, derived from ices or gained by other means, that can be transported and manipulated to change their energetic form (phase) through the combination of pressure and temperature. Newer versions now have gasses and liquids handled completely separately. | |
| − | + | This is opposed to solid resources, processed irreversibly from ores into pure ingots and alloys, used directly for crafting purposes. Gases have a great variety of uses - some are required for creation of livable pressurized environments, while others are toxic and dangerous and meant for controlled industrial processes, or outright waste product with limited utility. | |
| − | + | Naturally, gases can exist as a constituence of a planetary atmosphere, available in a virtually infinite amount and possible to capture through the use of appropriate atmospheric devices, can be pressurized, filtered, and stored. Planets or moons that have a below-zero or vacuum conditions will also contain frozen gas deposits in a form of ice, which can be melted by the use of a [[Furnace]] or [[Ice Crusher]]. Also, mined ice nuggets appear similar to metallic ores, however they will begin to melt if exposed to a warm environment while held in arms or regular containers. | |
| − | + | ||
| − | + | Small amount of gases can be acquired from smelting solid materials into ingots, as each type of ore contains its own impurities, although these gases mostly seen as an impediment to efficient smelting due to low output.<br> | |
| − | * [[Special:MyLanguage/ | + | Finally, gases can be purchased from [[traders]] in various forms and at different temperatures. Some traders will also buy gases and mixtures that they need with certain temperature conditions, or even pay you a small fee for disposing of their own waste gases. |
| − | * [[Special:MyLanguage/Nitrogen|Nitrogen (N<sub>2</sub>)]] | + | |
| − | * [[Special:MyLanguage/Nitrous Oxide|Nitrous Oxide (N<sub>2</sub>O)]] | + | Current variety of gases include: |
| − | * [[Special:MyLanguage/Oxygen|Oxygen (O<sub>2</sub>)]] | + | * [[Special:MyLanguage/Carbon Dioxide|Carbon Dioxide (CO<sub>2</sub>)]] - Inert gas, a non-toxic byproduct of burning organic fuels, breathed by most growing plants as source of Carbon. |
| − | * [[Special:MyLanguage/Pollutant|Pollutants (X)]] | + | * [[Special:MyLanguage/Nitrogen|Nitrogen (N<sub>2</sub>)]] - Heavy gas, usable as a stable pressure agent due to its exceptionally low freezing temperature. |
| − | * [[Special:MyLanguage/Volatiles|Volatiles]] | + | * [[Special:MyLanguage/Nitrous Oxide|Nitrous Oxide (N<sub>2</sub>O)]] - Commonly known as "Nitro", a loose chemical composite of Nitrogen and Oxygen, used as powerful fuel oxidizer and general anesthetic. |
| − | * [[Special:MyLanguage/Water|Water (H<sub>2</sub>O)]] | + | * [[Special:MyLanguage/Oxygen|Oxygen (O<sub>2</sub>)]] - Highly-reactive gas used for respiration by animal life and as conventional fuel oxidizer. |
| + | * [[Special:MyLanguage/Pollutant|Pollutants (X)]] - Ambiguous compound of highly-toxic radicals, a waste byproduct from burning or processing of impure organic fuel. | ||
| + | * [[Special:MyLanguage/Volatiles|Volatiles (CH<sub>4</sub>)+]] - Generic composite of simple organic compounds used as a fuel in smelting, power generation, and chemistry. | ||
| + | * [[Special:MyLanguage/Water|Water (H<sub>2</sub>O)]] - Pure compound used for hydration, farming, and as coolant, originally designed as inherently liquid substance. | ||
</translate> | </translate> | ||
| + | ==Properties== | ||
| + | Gases and their liquid analogues are presented in the parametric form - that means that they exist as a property of a given atmosphere' grid cell, interior grid cell of an enclosed room, composite [[pipe]] network, atmospheric device, or portable container. To further understand the behavior of gases within different spaces, check [[Atmosphere]] and [[Pipe]] pages. It is technically possible to convert gases down into their solid state (i.e. a physical item), however that action has limited practical use, beyond perhaps hand measuring of specific quantities, and no dedicated devices exist for doing so. | ||
| + | |||
| + | Each different gas have a number of properties, which define its thermodynamic behavior and conditions for phase changes. Leveraging the property values and their differences between gases is an important part of atmospheric management and temperature conditioning required for surviving extreme environments and utilizing advanced technologies: | ||
| + | * '''Melting Point''' - Temperature boundary between solid and liquid states. Liquids cooled to this temperature will begin freezing. This will create ice chunks converting up to 50 mol of liquid into a 50g stack of pure ice. Liquids freezing within containers or pipes will cause them to burst irreversibly. A player should make sure never to cool the gas down to this value. | ||
| + | |||
| + | * '''Boiling Point''' - Temperature boundary between liquid and gaseous states at standard 1 atm (100 kPa) of pressure. Higher pressures impede evaporation, raising this value and allowing to form liquids at higher temperatures. Most atmospheric devices are designed to work with gases, while liquified substances are very compact. Player should control pressure and temperature to prevent gases and liquids reaching this value unintentionally. Not available for substances, that will remain in gaseous phase at 100 kPa regardless of temperature. | ||
| + | |||
| + | * '''Minimum Condensation Pressure''' - a Pressure value corresponding to the Melting Point Temperature, which is necessary for the liquid to begin freezing. Most liquified gases will freeze up when cooled below their melting point regardless, but for ''some'' gases a low pressure environment can be sustained to keep a liquid in a super-cooled state without allowing it to crystallize, which is useful for storage of few particular substances. | ||
| + | |||
| + | * '''Maximum Liquid Temperature''' - a Temperature value, above which the substance cannot remain in liquid state within working pressure range, usually sampled at high pressure (6 MPa). Exponentially higher pressures are capable of keeping the liquid from evaporating, but the requirements are unrealistic for physical limitations of Stationeers' equipment. Its a practical heat boundary, under which a given substance can be either gas, liquid, or both based on the imposed pressure level, all the way down to the melting point boundary. | ||
| + | * '''Molar Heat Capacity''' - The thermal capacity of 1 mol of the gas, that it has to absorb/radiate to raise/lower its temperature by 1 K. The greater this value, the more heat a cold gas can absorb to cool another gas, and inversely, the more heat has to be drawn out to cool it. Particularly important value for an efficient coolant. | ||
| − | == | + | * '''Molar Heat of Fusion''' - The amount of thermal energy that a mol of frozen substance would need to absorb to change into a liquid, and the amount of same energy a liquid will release into environment when turning into ice. Given that frozen substances are rarely used as anything beyond a raw source material for gases, this value has little importance. |
| + | |||
| + | * '''Molar Latent Heat''' - The amount of thermal energy that a mol of liquid substance would need to absorb to change into a gas, and the amount of same energy a gas would release when turning into a liquid. This value is something that is often leveraged in AC or any temperature control systems, by forcing phase changes at specific points of a system through applied pressure. It is also likely responsible for the majority of confusing and frustrating emergencies for inexperienced Stationeers, where temperatures suddenly spike or drop to dangerous levels, seemingly for no reason. | ||
| + | |||
| + | * '''Molar Liquid Volume''' - Volume in Liters that a mol of substance occupy in liquid form. Since liquified gases produce no Pressure, this value defines how much of a given liquified gas can be stored in a liquid container or pipe network of a certain capacity. | ||
| + | |||
| + | * '''Molar Mass''' - Net mass of a mol of substance in any phase state. Largely redundant parameter that becomes important when dealing in Rocketry, since the rocket engines have specific limits to what mass they're able to carry into space against a local gravity, and excessive amount of fuel/payload can make a lift-off impossible. | ||
| + | |||
| + | == Phase Change == | ||
| + | The summary of the aforementioned properties define how a given gas behaves depending on the temperature and pressure at which it is contained. By accessing in-game Stationpedia, a player can find a phase diagram for any such gas in a logarithmic scale. A more readable version for all gases is here:<br> | ||
| + | <br> | ||
| + | |||
| + | [[File:Gases-phase-change-diagram.png|1200px|center|Gases diagram]] | ||
| + | <br> | ||
| + | |||
| + | Interactive version: [https://gralewski.github.io/stationeers-gas-phase-diagram-by-zgralewski.htm Here], Reddit post: [https://www.reddit.com/r/Stationeers/comments/1pzrojd/comment/nwtdvrh/ Here] | ||
| + | |||
| + | The left part of each diagram correlates to substance's solid (ice) state. A vertical blue cut-off indicates its melting point. | ||
| + | Next goes the dynamic curve (main section), which separates the possible conditions for a liquid state (above the curve) and a gas state (below the curve), the curve itself being the phase change boundary between the two. | ||
| + | Finally, the curve terminates by an abrupt vertical spike - this is a Maximum Liquid Temperature boundary, after which the substance will almost always remain as gas. | ||
| + | |||
| + | A more complex phase change diagram can be located [https://www.desmos.com/calculator/voj4scjipd Here] | ||
| + | |||
| + | Known gas properties: | ||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
| − | ! Name !! Filter Color !! Molar Heat Capacity ( | + | ! Name !! Filter Color !! Molar Heat Capacity<br>(J⋅K<sup>−1</sup>⋅mol<sup>−1</sup>) !! Molar Heat of Fusion<br>(kJ⋅K<sup>−1</sup>⋅mol<sup>−1</sup>) !! data-sort-type="number" | Melting Point<br>(K / °C) !! data-sort-type="number" | Boiling Point at 100 kPa<br>(K / °C) !! data-sort-type="number" | Minimum Condensation<br>(kPa at K / °C) !! data-sort-type="number" | Maximum Liquid Temperature<br>(K / °C at kPa) !! Molar Latent Heat<br> (kJ / mol) !! Liquid Molar Volume<br>(L / mol) !! Molar Mass<br>(g / mol) |
|- | |- | ||
| − | | [[Carbon Dioxide]] (CO<sub>2</sub>) || Gray || 28.2 || 0.6 || 218 / -55.3 || N/A || 517 at 218 / -55.3 || 265 / -8.1 at 6000 | + | | [[Carbon Dioxide]] (CO<sub>2</sub>) || Gray || 28.2 || 0.6 || 218 / -55.3 || N/A || 517 at 218 / -55.3 || 265 / -8.1 at 6000 || 0.6 || 0.04 || 44 |
|- | |- | ||
| − | | [[Nitrogen]] (N<sub>2</sub>) || Black || 20.6 || 0.5 || 40 / -233 || 75 / -198 || 6.3 at 40 / 233 || 190 / -83 at 6000 | + | | [[Nitrogen]] (N<sub>2</sub>) || Black || 20.6 || 0.5 || 40 / -233 || 75 / -198 || 6.3 at 40 / -233 || 190 / -83 at 6000 || 0.5 || 0.0348 || 64<!--That's correct on ingame files as of 0.2.6091.26702, can be looked up on Scripts.Atmospherics.Mole:MolarMass()--> |
|- | |- | ||
| − | | [[Nitrous Oxide]] (N<sub>2</sub>O) || Green || 37.2 || 4 || 252 / -21 || N/A || 800 at 252 / -21 || 431 / 158 at 2000 | + | | [[Nitrous Oxide]] (N<sub>2</sub>O) || Green || 37.2 || 4 || 252 / -21 || N/A || 800 at 252 / -21 || 431 / 158 at 2000 || 4 || 0.026 || 46 |
|- | |- | ||
| − | | [[Oxygen]] (O<sub>2</sub>) || White || 21.1 || 0.8 || 56.4 / -217 || 90 / -183 || 6.3 at 56.4 / -217 || 162 / -111 at 6000 | + | | [[Oxygen]] (O<sub>2</sub>) || White || 21.1 || 0.8 || 56.4 / -217 || 90 / -183 || 6.3 at 56.4 / -217 || 162 / -111 at 6000 || 0.8 || 0.03 || 16 |
|- | |- | ||
| − | | [[Pollutant]] (X) || Yellow || 24.8 || 2 || 173 / -99.8 || N/A || 1800 at 173 / -99.8 || 425 / 152 at 6000 | + | | [[Pollutant]] (X) || Yellow || 24.8 || 2 || 173 / -99.8 || N/A || 1800 at 173 / -99.8 || 425 / 152 at 6000 || 2 || 0.04 || 28 |
|- | |- | ||
| − | | [[Volatiles]] (H<sub>2</sub>) || Red || 20.4 || 1 || 81.6 / -192 || 112 / -162 || 6.3 at 81.6 / -192 || 195 / -78.1 at 6000 | + | | [[Volatiles]] (H<sub>2</sub>) || Red || 20.4 || 1 || 81.6 / -192 || 112 / -162 || 6.3 at 81.6 / -192 || 195 / -78.1 at 6000 || 1 || 0.04 || 16 |
|- | |- | ||
| − | | [[Water]] (H<sub>2</sub>0) || Blue || 72 || 8 || 273 / 0 || 373 / 100 || 6.3 at 273 / 0 || 643 / 370 at 6000 | + | | [[Water]] (H<sub>2</sub>0) || Blue || 72 || 8 || 273 / 0 || 373 / 100 || 6.3 at 273 / 0 || 643 / 370 at 6000 || 8 || 0.018 || 18 |
|} | |} | ||
| − | |||
| − | |||
| − | |||
==Mixtures== | ==Mixtures== | ||
| + | While the gases can exist in any arbitrary number of combinations, there are specific functional mixtures produced manually - this is done by filtering and isolating individual component gases and then mixing them in proper ratio using a [[Gas Mixer]]. Such specific mixtures are commonly referenced by their separate names for all associated cases, without mentioning their constituents: | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
! Name !! Contents !! Tank Color !! Notes | ! Name !! Contents !! Tank Color !! Notes | ||
|- | |- | ||
| − | | [[Fuel]] || 67% H<sub>2</sub>, 33% O<sub>2</sub> || Orange || Autoignition at 573.15 K / 300 °C | + | | [[Fuel]] || 67% H<sub>2</sub>, 33% O<sub>2</sub> || Orange || Combustible mixture with Autoignition at 573.15 K / 300 °C |
|- | |- | ||
| − | | [[HydroNox]] || 50% H<sub>2</sub>, 50% N<sub>2</sub>O || N/A || Autoignition at 323.15 K / 50 °C | + | | [[HydroNox]] / "Superfuel" || 50% H<sub>2</sub>, 50% N<sub>2</sub>O || N/A || Combustible mixture with Autoignition at 323.15 K / 50 °C |
|- | |- | ||
| − | | [[Air]] || 75% N<sub>2</sub>, 25% O<sub>2</sub> || White || 16 kPa | + | | [[Air]] || 75% N<sub>2</sub>, 25% O<sub>2</sub> || White || Well above 16 kPa of minimum O<sub>2</sub> pressure required for human breathing, but prevents runaway combustion by spark) |
|} | |} | ||
==Simulation Mechanics== | ==Simulation Mechanics== | ||
| − | + | {| class="wikitable" | |
| − | + | |- | |
| − | + | ! Concept | |
| − | + | ! Simulated | |
| − | + | ! Notes | |
| − | + | |- | |
| − | + | | Ideal Gas Law (PV=nRT) | |
| − | + | | Partial | |
| − | + | | Pressure will change with temperature, volume, and mols; temperature will not change with pressure, volume, or mols | |
| − | + | |- | |
| − | + | | Adiabatic Heating and Cooling | |
| + | | No | ||
| + | | Compressing or expanding a gas will not affect its temperature or the temperature around it | ||
| + | |- | ||
| + | | Thermal Conductivity | ||
| + | | No | ||
| + | | Rate of energy transfer is the same for all substances | ||
| + | |- | ||
| + | | Thermal Radiation | ||
| + | | Partial | ||
| + | | Radiated heat is not absorbed by nearby objects | ||
| + | |- | ||
| + | | Phase Change | ||
| + | | Partial | ||
| + | | Gas/Liquid have simplified graph; Solid has a fixed temperature; Plasma is ignored | ||
| + | |- | ||
| + | | Fluid Dynamics | ||
| + | | No | ||
| + | | 'Convection' parts work in pressurized environment, other parts work in vacuum | ||
| + | |- | ||
| + | | Gas Stoichiometry | ||
| + | | No | ||
| + | | The various reactions are arbitrary and vary depending on device used for different products from the same reactants | ||
| + | |- | ||
| + | | Gravity/Density | ||
| + | | No | ||
| + | | Gas/Liquid is present in all directions | ||
| + | |- | ||
| + | | Environment Volume | ||
| + | | Yes | ||
| + | | Each environment cube acts as a single point with fixed volume | ||
| + | |- | ||
| + | | Saturation | ||
| + | | No | ||
| + | | Mixture and % do not affect condensation/evaporation | ||
| + | |- | ||
| + | | Pipe Volume | ||
| + | | Yes | ||
| + | | Each pipe network acts as a single point with fixed volume depending on number of pipes segments | ||
| + | |- | ||
| + | | Flow Direction | ||
| + | | Partial | ||
| + | | All pumps and mixer act as check valve, passive check valve available, other valves are not one-way; no flow within each connected pipe network | ||
| + | |- | ||
| + | | Pipe Integrity | ||
| + | | Yes | ||
| + | | Liquid pipes hold low pressure, but may contain both liquid and gas without consequence; Gas pipes hold high pressure, but stress and break with too much liquid | ||
| + | |- | ||
| + | | Heat Transfer | ||
| + | | Partial | ||
| + | | Liquid temperature is controlled by gas temperature (if no gas, liquid heater will not function) | ||
| + | |} | ||
| + | |||
| + | [[Category:Gas]][[Category:Liquid]] | ||
Latest revision as of 06:19, 1 February 2026
In older versions of Stationeers, Gas was a generalized term for fluid substances, derived from ices or gained by other means, that can be transported and manipulated to change their energetic form (phase) through the combination of pressure and temperature. Newer versions now have gasses and liquids handled completely separately.
This is opposed to solid resources, processed irreversibly from ores into pure ingots and alloys, used directly for crafting purposes. Gases have a great variety of uses - some are required for creation of livable pressurized environments, while others are toxic and dangerous and meant for controlled industrial processes, or outright waste product with limited utility.
Naturally, gases can exist as a constituence of a planetary atmosphere, available in a virtually infinite amount and possible to capture through the use of appropriate atmospheric devices, can be pressurized, filtered, and stored. Planets or moons that have a below-zero or vacuum conditions will also contain frozen gas deposits in a form of ice, which can be melted by the use of a Furnace or Ice Crusher. Also, mined ice nuggets appear similar to metallic ores, however they will begin to melt if exposed to a warm environment while held in arms or regular containers.
Small amount of gases can be acquired from smelting solid materials into ingots, as each type of ore contains its own impurities, although these gases mostly seen as an impediment to efficient smelting due to low output.
Finally, gases can be purchased from traders in various forms and at different temperatures. Some traders will also buy gases and mixtures that they need with certain temperature conditions, or even pay you a small fee for disposing of their own waste gases.
Current variety of gases include:
- Carbon Dioxide (CO2) - Inert gas, a non-toxic byproduct of burning organic fuels, breathed by most growing plants as source of Carbon.
- Nitrogen (N2) - Heavy gas, usable as a stable pressure agent due to its exceptionally low freezing temperature.
- Nitrous Oxide (N2O) - Commonly known as "Nitro", a loose chemical composite of Nitrogen and Oxygen, used as powerful fuel oxidizer and general anesthetic.
- Oxygen (O2) - Highly-reactive gas used for respiration by animal life and as conventional fuel oxidizer.
- Pollutants (X) - Ambiguous compound of highly-toxic radicals, a waste byproduct from burning or processing of impure organic fuel.
- Volatiles (CH4)+ - Generic composite of simple organic compounds used as a fuel in smelting, power generation, and chemistry.
- Water (H2O) - Pure compound used for hydration, farming, and as coolant, originally designed as inherently liquid substance.
Properties[edit]
Gases and their liquid analogues are presented in the parametric form - that means that they exist as a property of a given atmosphere' grid cell, interior grid cell of an enclosed room, composite pipe network, atmospheric device, or portable container. To further understand the behavior of gases within different spaces, check Atmosphere and Pipe pages. It is technically possible to convert gases down into their solid state (i.e. a physical item), however that action has limited practical use, beyond perhaps hand measuring of specific quantities, and no dedicated devices exist for doing so.
Each different gas have a number of properties, which define its thermodynamic behavior and conditions for phase changes. Leveraging the property values and their differences between gases is an important part of atmospheric management and temperature conditioning required for surviving extreme environments and utilizing advanced technologies:
- Melting Point - Temperature boundary between solid and liquid states. Liquids cooled to this temperature will begin freezing. This will create ice chunks converting up to 50 mol of liquid into a 50g stack of pure ice. Liquids freezing within containers or pipes will cause them to burst irreversibly. A player should make sure never to cool the gas down to this value.
- Boiling Point - Temperature boundary between liquid and gaseous states at standard 1 atm (100 kPa) of pressure. Higher pressures impede evaporation, raising this value and allowing to form liquids at higher temperatures. Most atmospheric devices are designed to work with gases, while liquified substances are very compact. Player should control pressure and temperature to prevent gases and liquids reaching this value unintentionally. Not available for substances, that will remain in gaseous phase at 100 kPa regardless of temperature.
- Minimum Condensation Pressure - a Pressure value corresponding to the Melting Point Temperature, which is necessary for the liquid to begin freezing. Most liquified gases will freeze up when cooled below their melting point regardless, but for some gases a low pressure environment can be sustained to keep a liquid in a super-cooled state without allowing it to crystallize, which is useful for storage of few particular substances.
- Maximum Liquid Temperature - a Temperature value, above which the substance cannot remain in liquid state within working pressure range, usually sampled at high pressure (6 MPa). Exponentially higher pressures are capable of keeping the liquid from evaporating, but the requirements are unrealistic for physical limitations of Stationeers' equipment. Its a practical heat boundary, under which a given substance can be either gas, liquid, or both based on the imposed pressure level, all the way down to the melting point boundary.
- Molar Heat Capacity - The thermal capacity of 1 mol of the gas, that it has to absorb/radiate to raise/lower its temperature by 1 K. The greater this value, the more heat a cold gas can absorb to cool another gas, and inversely, the more heat has to be drawn out to cool it. Particularly important value for an efficient coolant.
- Molar Heat of Fusion - The amount of thermal energy that a mol of frozen substance would need to absorb to change into a liquid, and the amount of same energy a liquid will release into environment when turning into ice. Given that frozen substances are rarely used as anything beyond a raw source material for gases, this value has little importance.
- Molar Latent Heat - The amount of thermal energy that a mol of liquid substance would need to absorb to change into a gas, and the amount of same energy a gas would release when turning into a liquid. This value is something that is often leveraged in AC or any temperature control systems, by forcing phase changes at specific points of a system through applied pressure. It is also likely responsible for the majority of confusing and frustrating emergencies for inexperienced Stationeers, where temperatures suddenly spike or drop to dangerous levels, seemingly for no reason.
- Molar Liquid Volume - Volume in Liters that a mol of substance occupy in liquid form. Since liquified gases produce no Pressure, this value defines how much of a given liquified gas can be stored in a liquid container or pipe network of a certain capacity.
- Molar Mass - Net mass of a mol of substance in any phase state. Largely redundant parameter that becomes important when dealing in Rocketry, since the rocket engines have specific limits to what mass they're able to carry into space against a local gravity, and excessive amount of fuel/payload can make a lift-off impossible.
Phase Change[edit]
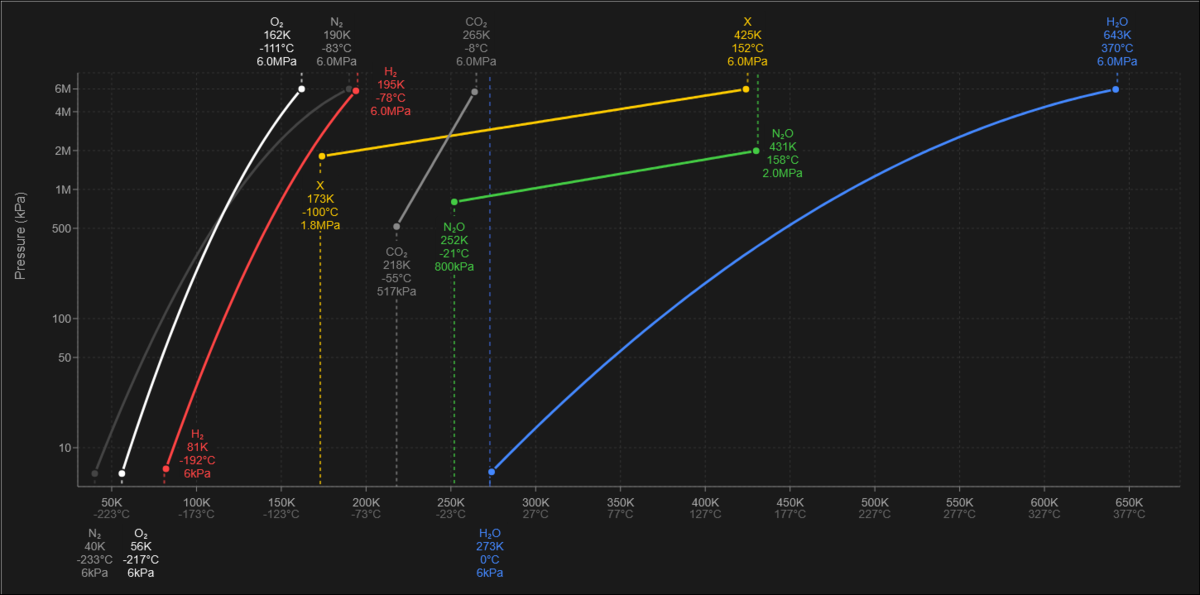
The summary of the aforementioned properties define how a given gas behaves depending on the temperature and pressure at which it is contained. By accessing in-game Stationpedia, a player can find a phase diagram for any such gas in a logarithmic scale. A more readable version for all gases is here:
Interactive version: Here, Reddit post: Here
The left part of each diagram correlates to substance's solid (ice) state. A vertical blue cut-off indicates its melting point. Next goes the dynamic curve (main section), which separates the possible conditions for a liquid state (above the curve) and a gas state (below the curve), the curve itself being the phase change boundary between the two. Finally, the curve terminates by an abrupt vertical spike - this is a Maximum Liquid Temperature boundary, after which the substance will almost always remain as gas.
A more complex phase change diagram can be located Here
Known gas properties:
| Name | Filter Color | Molar Heat Capacity (J⋅K−1⋅mol−1) |
Molar Heat of Fusion (kJ⋅K−1⋅mol−1) |
Melting Point (K / °C) |
Boiling Point at 100 kPa (K / °C) |
Minimum Condensation (kPa at K / °C) |
Maximum Liquid Temperature (K / °C at kPa) |
Molar Latent Heat (kJ / mol) |
Liquid Molar Volume (L / mol) |
Molar Mass (g / mol) |
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon Dioxide (CO2) | Gray | 28.2 | 0.6 | 218 / -55.3 | N/A | 517 at 218 / -55.3 | 265 / -8.1 at 6000 | 0.6 | 0.04 | 44 |
| Nitrogen (N2) | Black | 20.6 | 0.5 | 40 / -233 | 75 / -198 | 6.3 at 40 / -233 | 190 / -83 at 6000 | 0.5 | 0.0348 | 64 |
| Nitrous Oxide (N2O) | Green | 37.2 | 4 | 252 / -21 | N/A | 800 at 252 / -21 | 431 / 158 at 2000 | 4 | 0.026 | 46 |
| Oxygen (O2) | White | 21.1 | 0.8 | 56.4 / -217 | 90 / -183 | 6.3 at 56.4 / -217 | 162 / -111 at 6000 | 0.8 | 0.03 | 16 |
| Pollutant (X) | Yellow | 24.8 | 2 | 173 / -99.8 | N/A | 1800 at 173 / -99.8 | 425 / 152 at 6000 | 2 | 0.04 | 28 |
| Volatiles (H2) | Red | 20.4 | 1 | 81.6 / -192 | 112 / -162 | 6.3 at 81.6 / -192 | 195 / -78.1 at 6000 | 1 | 0.04 | 16 |
| Water (H20) | Blue | 72 | 8 | 273 / 0 | 373 / 100 | 6.3 at 273 / 0 | 643 / 370 at 6000 | 8 | 0.018 | 18 |
Mixtures[edit]
While the gases can exist in any arbitrary number of combinations, there are specific functional mixtures produced manually - this is done by filtering and isolating individual component gases and then mixing them in proper ratio using a Gas Mixer. Such specific mixtures are commonly referenced by their separate names for all associated cases, without mentioning their constituents:
| Name | Contents | Tank Color | Notes |
|---|---|---|---|
| Fuel | 67% H2, 33% O2 | Orange | Combustible mixture with Autoignition at 573.15 K / 300 °C |
| HydroNox / "Superfuel" | 50% H2, 50% N2O | N/A | Combustible mixture with Autoignition at 323.15 K / 50 °C |
| Air | 75% N2, 25% O2 | White | Well above 16 kPa of minimum O2 pressure required for human breathing, but prevents runaway combustion by spark) |
Simulation Mechanics[edit]
| Concept | Simulated | Notes |
|---|---|---|
| Ideal Gas Law (PV=nRT) | Partial | Pressure will change with temperature, volume, and mols; temperature will not change with pressure, volume, or mols |
| Adiabatic Heating and Cooling | No | Compressing or expanding a gas will not affect its temperature or the temperature around it |
| Thermal Conductivity | No | Rate of energy transfer is the same for all substances |
| Thermal Radiation | Partial | Radiated heat is not absorbed by nearby objects |
| Phase Change | Partial | Gas/Liquid have simplified graph; Solid has a fixed temperature; Plasma is ignored |
| Fluid Dynamics | No | 'Convection' parts work in pressurized environment, other parts work in vacuum |
| Gas Stoichiometry | No | The various reactions are arbitrary and vary depending on device used for different products from the same reactants |
| Gravity/Density | No | Gas/Liquid is present in all directions |
| Environment Volume | Yes | Each environment cube acts as a single point with fixed volume |
| Saturation | No | Mixture and % do not affect condensation/evaporation |
| Pipe Volume | Yes | Each pipe network acts as a single point with fixed volume depending on number of pipes segments |
| Flow Direction | Partial | All pumps and mixer act as check valve, passive check valve available, other valves are not one-way; no flow within each connected pipe network |
| Pipe Integrity | Yes | Liquid pipes hold low pressure, but may contain both liquid and gas without consequence; Gas pipes hold high pressure, but stress and break with too much liquid |
| Heat Transfer | Partial | Liquid temperature is controlled by gas temperature (if no gas, liquid heater will not function) |