Phase Change Mechanics
From Unofficial Stationeers Wiki
| This page is a work-in-progress, most of the information here will hold true until a later patch or revision may invalidate or update information |
The Phase Change Major update added phases to all the existing gases and liquids. Before a gas pipe would hold all the gases except for water, but now all the gases are capable of existing as a solid, liquid, or a gas. This added complexity can further make an inhospitable world even crueler, but once tamed can make your stay just a little more welcome.
This guide will explain the major changes the update brings, as well as how some of the thermodynamics work to those who are not familiar. As such use this page as a general guide for explaining concepts that may be confusing or unfamiliar.
Contents
MAJOR CHANGES[edit]
These are the major changes the update brings
- All gases : Carbon Dioxide, Oxygen, Pollutants, Nitrogen, Volatiles, and Nitrous Oxides and Liquids : Water will now be capable of existing in either a gas, liquid, and/or solid state depending on Temperature and Pressure
- Gas Pipe volumes have been reduced by a factor of 10, Volume pumps have also been reduced in pumping capacity by the same factor.
- Liquid pipes now need to be pressurized to hold liquids.
- 2 Phase Change devices: Condensation Chamber and Evaporation Chamber
- In-Line tanks for both Gas and Liquid pipe systems. These will also include the Gas Tank Storage and Liquid Tank Storage Respectively.
- Passive Liquid inlets for safely removing liquids from the world into its connected pipe network.
- Expansion Valve and Condensation Valve variants to the Pipe Valve Kit
- Pressurant Valve and Purge Valve to the Pressure Regulator Kit
Phase Change Basics[edit]
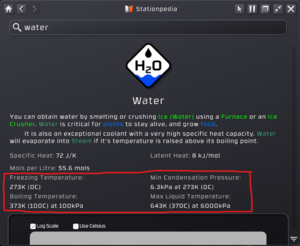
Best way to explain the basics is to recall how water behaves in real life. Depending on the temperature water can freeze to become ice if the temperature drops below 0 C (32 F). Water also evaporates into steam when it is above 100 C (212F). All gases in stationeers will have their own state of phases at varying pressures and temperatures and will be noted in the stationpedia entry for every one of them. One thing to remember with the example with water is that the pressure of water is understood to be 1 atm (approximately 101 k PA) of air pressure. As the pressure rises or falls, the gas will have different condensation or evaporation points. As such every stationpedia entry will have absolute freezing points and maximum condensation points @ exactly 6 MPA of pressure.
Phase Change Graph[edit]
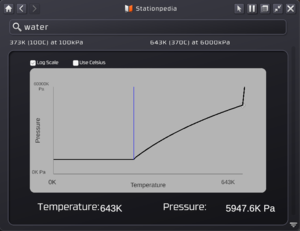
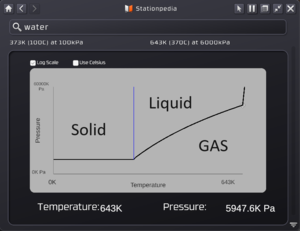
Every gas will have a phase change diagram to better show the relationship of Temperature vs Pressure. The picture will show you how to read it for any gas you look up:
The diagram is a line-curve of Pressure vs Temperature. Left to Right is increasing temperature and from bottom to top is increasing pressure. The boxes for log scale or Celcius just change how the information is displayed. The solid blue line is the freezing point of the gas (for water it is 0C or 273k). To the left of that line, or basically temperatures below that point means water will freeze. Above that temperature and above the curve is the condensation point of the gas or when it will condense to a liquid. This is completely dependent on the Temperature and pressure. As you can see, at a higher pressure, you will need a higher temperature to keep condensation from happening. So long as the gas is below the curve, it will stay as a gas. The hard limit is 6000 kpa or 6 MPA as that is the limit a liquid pipe will hold.
One huge benefit of the stationpedia is as you mouse over the graph, you will have a small cursor show you the exact temperature and pressure of the curve at that point, allowing you to very easily figure out a key information for what the condensation point is. As you look at the above screenshots when they were taken, the cursor was hovering over to find out that at 643K (370C) you need a pressure of 5947.6 kpa to keep water as a liquid (functions as both a condensation and evaporation point, more on that in the following section.)
Phase Changing[edit]
Now that you can read the diagram, you can now understand what happens during a phase change. The two key terms are Condensation and Evaporation.
When Condensation Occurs, a gas goes from a higher energy state to a lower energy state. Some of the gas transforms to a liquid, and in so doing heat is released to its surroundings/container to complete the transformation.
When Evaporation Occurs, a liquid goes from a lower energy state to a higher energy state. Some of the liquid transforms to a gas, and in so doing absorbs heat from its surroundings/containter to complete the transformation.
Why this occurs is also a simple process. No matter what is in a container, gases and liquids want to stay at a stable, equilibrium state based on its Temperature and how dense the atmosphere is in its container (Pressure). If we take our liquid water at 100kpa, it will evaporate at 100C because the atmosphere (100kpa) can only exert enough force to keep water as a liquid so long as it is under 100C. As soon as the liquid water's temperature goes past that point, some of the mols of water escapes the liquid state and evaporates into the atmosphere. This will cause less water molecules to be in the liquid state, some will now be in the atmosphere, and the temperature of the atmosphere will decrease as a result. Since the atmosphere now also has steam, the pressure will increase. All this is done in an attempt to keep whatever water is left as liquid to stay as a liquid. Stationeers simulates the contents of its atmosphere/pipes as a single unit. Unlike real life, we would have had the temperature of the water and the temperature of the atmosphere it is exposed to as two separate temperatures.
To better explain phase changing, I will present situations under the following assumptions: #1, the contents of the atmosphere do not matter and will thus change to match the pressures I stated and #2 this is an enclosed system such that none of the phase changing component will be lost.
With a container that holds exactly 100 Kpa of gas and some amount of liquid water. Initial condition is 20C
- Example #1: The temperature of the whole mixture is increased until 130C is reached. Assume the pressure stays constant 100 kPA until phase change occurs.
- Results: Some of the liquid water evaporates, this will add some molecules of water to its atmosphere to increase the pressure. In turn likewise the evaporation will attempt to cool the temperature down of its surroundings. Net result since the container is heated to exactly 130C, we will be left with a container pressurized to 144 kpa.
- Example #2: 20C steam (water in a gas state) is added to the initial mixture.
- Results: This steam will condense as soon as it is added to this container since 20C < 100C. The condensation occurring with the steam will cause the temperature of the mixture to begin to increase.
- Example #3: You decide to remove the pressurizing air for the initial mixture. At what pressure will you begin to see evaporation of the 20C water?
- Results: When the pressure drops below 11.5 kpa you will begin to see water evaporate. This will also lower the temperature of the water if evaporation begins occurring.
- Example #4: You have a container filled with 3000Kpa mixture of some gas + water @ 350C. You begin to cool this mixture, at what temperature will you expect the water to begin condensing? Assume the drop in temperature will not change the pressure until phase change occurs.
- Results: At about 317C is when this will begin condensing some of the water in the atmosphere. Realistically, the drop in temperature will also decrease the pressure in this container. You will see condensation occur at a lower temperature.
Hopefully the examples above will help explain how pressure and temperature affect when and if evaporation or condensation will occur. In short, low pressure or high temp means a liquid will evaporate. High Pressure or low temp means a gas will begin to condense to a liquid. As you move the mouse cursor across the phase change diagram, it will list you the specific temperature and pressure for that specific gas to begin condensing or when that liquid will evaporate.
Pipes and new kits[edit]
Gas and liquid pipes will be capable of holding gases, liquids, and/or solids; however, you must be careful about the contents. Gas Pipes can hold 60MPA of pressure difference of gases, but any liquids will stress the pipes. Gas Pipes will not take damage unless the stress is over 100%, which is when you have 1% of its volume worth of liquids in the gas pipe. Any ices in either pipe will rapidly deteriorate the pipe to bursting. Liquid Pipes can hold liquids safely, but can only be pressurized up to 6MPa. Pressure exceeding that will force the liquid pipe to start taking damage. Different gases have different temperature/pressure thresholds for being gases or liquids. You will need to adjust your method of storage.
World Considerations[edit]
Number 1 recommendation is to upgrade pipes to insulated pipes if they will be exposed to the world. This will ensure you dont have heat gain/loss that could cause damaging phase changes to your pipe network.
- Moon and Mimas: The vacuum nature will mean uninsulated pipes can cool to low temperatures to freeze any gas. The temperature change isn't fast unless you use radiators to radiate the heat, but be absolutely wary about which pipe networks will be exposed to the vacuum.
- Mars: Mars has a fairly temperate climate with low atmosphere. Be wary about CO2,NOS, and water being exposed to world conditions especially when it gets cold at night. The other gases will be more comfortable but be aware that pollutants will condense as you build them up in your tanks.
- Europa: Absolutely make sure nothing essential is exposed to the frigid air.
- Vulcan and Venus: The high temperatures mean your liquid piping is in danger of overpressurizing due to evaporation. High heat means it is hard to cool any gas down so do not let them heat up.
New Valves[edit]
The addition of the new valves will allow you to more easily move gases or liquids between your liquid and gas networks.
- Condensation Valve: Will move liquids from a gas network to a liquid network. Consideration: Ensure the liquid network is pressurized otherwhise this new liquid addition will evaporate and can rapidly cool to freezing.
- Expansion Valve: Will move Liquids from a liquid network to a gas network. Consideration: A pressurized gas network will mean these liquids will stay in your pipe network, be careful you dont burst your gas network with these liquids.
- Purge Valve: Will move gases from a liquid pipe network to a gas pipe network. Considerations: These work like a back pressure regulator and will remove excess gas pressure from the liquid pipe network. If you lower the liquid pipe network's pressure below its Temp-Press threshold, you will have existing liquids evaporate in the liquid pipe network.
- Pressurant Valve: Will move gases from a gas pipe network to a liquid pipe network. This is done to pressurize the liquid pipe network and this functions like a pressure regulator. If the pressure in the liquid pipe network exceeds what this gas's condensation point is, this gas will condense in the liquid pipe network.
Condensation and Expansion Valves are unpowered. Purge and Pressurant Valves require power to work.
Phase Change Devices[edit]
The Phase Change device comes in two variants: Condensation Chamber and Evaporation Chamber. The primary usage of these chambers is to safely hold phase change states of the gas within the chamber. They will have an input, output, and input 2 port for heat exchange (was called waste input but was changed recently). The device is the only one that can hold frozen gases without bursting. They have an open lever which if opened will cause the chamber to throw out all its contents into the surrounding atmosphere. They can both be set to a specific pressure.
Condensation Chambers have a gas input and liquid output. It will pull gases in from its gas input to reach the pressure setting. If any gases condense while attempting to reach that pressure it will push them out the liquid output. A gas pipe connected to input 2 can be used to exchange heat with its contents.
Evaporation Chambers have a liquid input and gas output. It will pull liquids in from its liquid input and allow them to evaporate. It will push out gases out to its gas output if the pressure within the chamber exceeds its pressure setting. Like the condensation chamber it can connect to a gas input 2 for heat exchange purposes.
How a stationeer will use these devices depends on the engineering solution needed. These can be used to make power efficient AC or Heat Pumps. They can also be used as a way to force liquids to evaporate or to control cool a gas to the point it condenses.
Final Words[edit]
With the above changes, controlling your atmosphere becomes that much more important. Each gas is treated as its own entity. Not only can your gases mix, but you can also have liquid mixtures. Stationeers may want to be careful about how they store and pressurize their pipe networks since it is now possible to have liquid NOS mixed into your liquid water system.
These basics should function unless something drastic is changed in a future patch. Mastering your gas atmospherics now involves taking into considerations when a phase change could occur. While most you will want to avoid, phase changes can increase your ability to move temperature around.