Phase Change guide
From Unofficial Stationeers Wiki
game version: 0.2.4297
Contents
- 1 Phase Change Update
- 1.1 How to read the in-game Stationpedia phase change diagrams
- 1.2 Valve and Pump behaviour
- 1.3 Liquid drain behaviour
- 1.4 Pipe damage
- 1.5 Separating a mix of gases or a mix of liquids
- 1.6 Dealing with extremely cold pipes
- 1.7 Heating / Cooling with phase change loops
- 1.8 Examples of how to take advantage of phase changes
- 1.9 Footnotes
Phase Change Update
In the Phase Change Update (july 2023) the 7 in-game gases (O2, N2, CO2, N2O, H2O, Volatiles and Pollutant) can now be in three different states; gas, liquid and solid. Gases and solids can exist by themselves, but a liquid will always co-exist with a gas (of any type). Phase changes are not instant, so it will take some time for them to stabilize.
How to read the in-game Stationpedia phase change diagrams
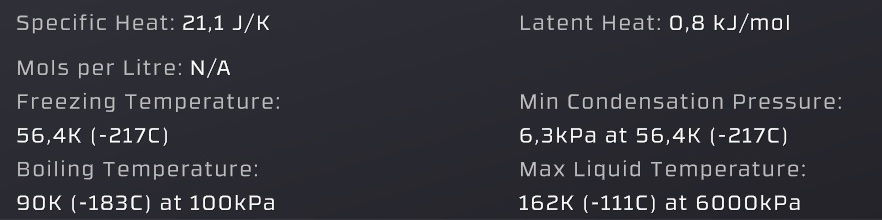
Specific heat = the energy per mol required to change the temperature by 1°C of both gas and liquid states
Freezing temperature = where a gas or liquid turns into solid (this is dangerous to pipes)
(unimportant) Boiling temperature = the temperature where the "Vapor pressure" is equal to 100kPa (slightly incorrect compared to in-game values)
Latent heat = the energy per mol that is released by condensation and consumed by evaporation
Min Condensation Pressure = below this pressure a gas will not condense into liquid (allows gases to freeze into solids)
Max liquid temperature = a gas can't condense into liquid above this temperature (gas-pipes are safe from liquid damage)
Vapor pressure = (the pressure value given by the graph in the liquid / gas range, in real life solids can have a vapor pressure too) a liquid evaporates when below it, a gas condensates when above it
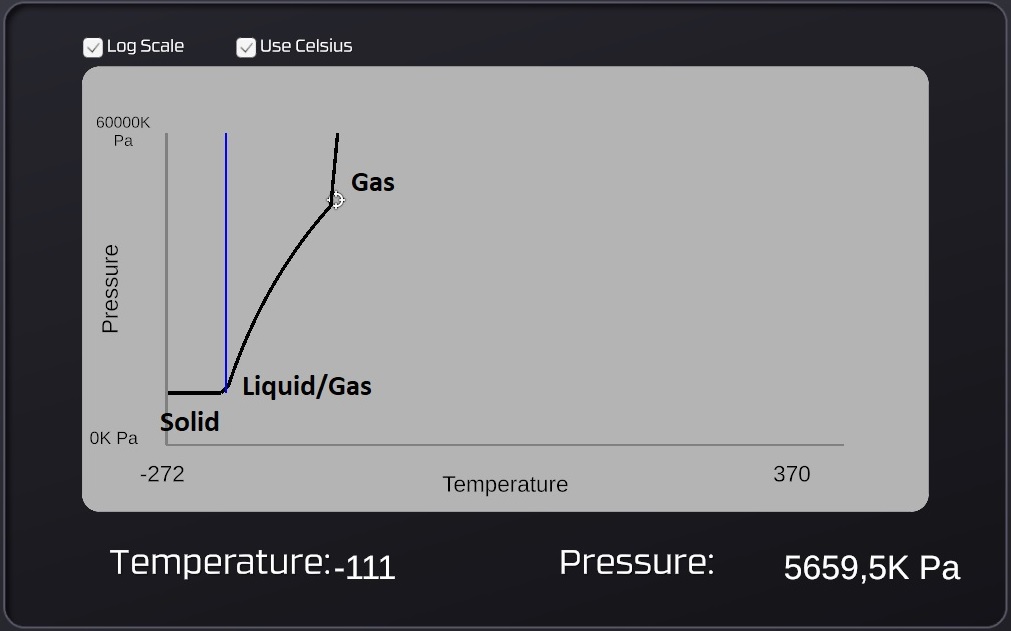
The Stationpedia graphs are divided into 3 different regions. Solid-state to the left, liquid / gas -state in the middle and the gas-state on the right. The term "Vapor pressure" isn't mentioned but is the pressure value that is read from the graph in the liquid / gas temperature range. This value is not completely accurate however, comparing the graph to in-game pressures show that they are slightly off (see the picture above: the -111°C is actually at 6000kPa), but it's always close enough to be helpful.
For solids the "Vapor pressure" is zero, even though the graph claims it to be 6.3 kPa (see footnote 1)
For liquids the "Vapor pressure" is the pressure where a liquid will stop evaporating. This pressure doesn't have to come from the same type of gas, another gas can also provide that pressure. Oxygen, Nitrogen and Volatiles are all good pressurants because they have low values for "Max liquid temperature" which makes them hard to turn into liquids (which would contaminate the liquid they are pressurizing).
For a gas with a temperature above the "Max liquid temperature" the "Vapor pressure" means nothing, because it's too hot to undergo any phase-changes in that range. But when the temperature drops below the "Max liquid temperature" (into the liquid / gas temperature range) the "Vapor pressure" becomes the lowest pressure required for a gas to start condensating. A gas can also turn into a solid (ice) directly without becoming a liquid first, this will happen if the temperature drops to the freezing point while the pressure of the gas is kept below the "Min Condensation Pressure". Example: Carbondioxide has a "Min Condensation Pressure" of 517kPa (at -55°C) and freezes at -55°C, so below 517kPa that gas will turn directly into a solid with a broken pipe as the result, this makes carbondioxide a risky (=fun) choice as a coolant gas despite its high capacity for absorbing heat (due to its high specific heat value).
Condensation is when a gas becomes liquid, this will release energy which increases the temperature.
Evaporation is when a liquid becomes gas, this will consume energy which decreases the temperature.
Freezing is when a gas or liquid becomes solid, this phase change does not appear to change the temperature.
Valve and Pump behaviour
(incomplete list, there are other objects with gas/liquid connections that could potentially move liquids and gases in useful ways)
Moves both gas and liquid
- Filtration unit
- Regulator
- Volume Pump
- Turbo Pump
- Valve
- One-way Valve
- Liquid Regulator
- Liquid Volume Pump
- Liquid Turbo Pump
- Liquid Valve
- Liquid One-way Valve
- Condensation Chamber (the gas-input)
Moves only gas
- Purge Valve (from liquid-pipes into gas-pipes)
- Pressurant Valve (from gas-pipes into liquid-pipes)
- Liquid Regulator with Setting = 0% (between liquid-pipes)
Moves only liquid
- Condensation Valve (from gas-pipes into liquid-pipes)
- Evaporation Valve (from liquid-pipes into gas-pipes)
- Evaporation Chamber (the liquid-input)
Liquid drain behaviour
- Passive Liquid Drain (placed on gas-pipes, dumps liquids into the atmosphere)
- Active Liquid Outlet (dumps liquids from liquid-pipes into the atmosphere)
- Passive Liquid Inlet (works like a Passive Vent but for liquid-pipes, gases can enter/exit and atmospheric liquids can enter)
Pipe damage
Gas pipes:
Takes damage from liquids if the "stress" value goes above 100%. They also take damage if an unknown amount of liquids or gas freezes into a solid.
Stress in % = 5000 x "liters of liquid" / "pipe network volume" (see footnote 2).
Liquid pipes:
Takes damage if the gas pressure goes above 6MPa, or if the liquid or gas freezes into a solid. The pipe volume is shared by both the gas and the liquid, so a liquid-pipe can become overpressurized by adding more liquid even though liquids themselves have no pressure.
Exploit:
Pipes inside double-welded frames will never take damage nor break from high pressure or stress. But they will still creak and moan.
Separating a mix of gases or a mix of liquids
Filtration: Filtration units can separate both gases and liquids, but too much liquid will damage the gas-pipes (unless using an exploit, see "Pipe damage" above).
Condensation: Condense a single type of gas into liquid from a gas mix when the temperature is below one of the gases "Max liquid temperature" and the pressure is above its "Vapor pressure" (performed in the Condensation Chamber or inside pipes/tanks).
Distillation: Evaporate a single type of liquid from a mix by keeping the gas pressure above the other liquids "Vapor pressure" and below the "Vapor pressure" of the liquid that is being removed (performed with the Evaporation Chamber or Purge Valve). An issue with distillation is that some pressurant gas must stay behind to prevent the remaining liquid from evaporating.
Separating a liquid from it's co-existing gas inside a liquid-pipe can be a bit tricky. This wiki author has only found three ways to do this, but there could be more.
A) Use an Evaporation Chamber to move only the liquid, then use heat to get that liquid out of that Chamber, then cool it down to get the liquid back.
B) Use an Evaporation Chamber with the handle on the front side open. This will dump all contents (liquids too) into the atmosphere around the Chamber. When this is done in a small (1x1x1) room, Passive Liquid Inlets can be used to recapture the dumped liquid.
C) An Evaporation Valve can move just the liquid out, but only through a gas-pipe so it's very easy to damage that gas-pipe (unless using an exploit, see "Pipe damage" above).
Examples:
1) (condensation) The atmosphere on Mars contains CO2, N2, O2 and Pollutant. At day-time temperatures, only the Pollutant will be below its "Max liquid temperature" (152°C at 6MPa) so it's the only gas that can condense into a liquid. Pressurizing atmospheric gas collected during the day (to roughly 4MPa or above) will force all of the Pollutant to condensate into liquid, a Passive Liquid Drain can then remove that liquid. The remaining gases are now free from toxins and safe to use in a greenhouse without further purification.
2) (distillation) A liquid mix of N2O and Pollutant can be separated via a distillation that only evaporates the Pollutant. When comparing the "Vapor pressures" of N2O and Pollutant one can see that for each temperature the Pollutant will always have the higher "Vapor pressure" value. With a Purge Valve or Evaporation Chamber it's possible to keep the pressure above the N2O's "Vapor pressure" and below the Pollutants, which means that only the Pollutant will be able to evaporate. When all the liquid Pollutant has been removed, only Pollutant gas and liquid N2O will remain, remove just the liquid phase.
Dealing with extremely cold pipes
When an AC is used for heating, its waste pipe can get extremely cold. The same goes for using passive Radiators on the Moon or on Mimas (keep the radiators exposed to sunlight to prevent the pipe temperature from getting too low). The best gas choice for cold pipes is Oxygen, since it is the hardest to liquify (-111°C at 6MPa), and all of it should (not properly tested!) turn into a liquid before it freezes into a solid. If the pipe pressure is above 6MPa the excess Oxygen will start to condense when the temperature dips to -111C. Attach a "Passive Liquid Drain" is a good way to protect the pipe, any liquid Oxygen will be removed from the pipe before it takes any damage. Instead of using pure Oxygen, crushed Oxite will do just fine, the Nitrogen will condense first and remove itself through the drain.
Heating / Cooling with phase change loops
Since condensation increases the temperature and evaporation decreases the temperature, it's possible to build a loop where the gas-side becomes warmer and the liquid-side colder. The Condensation and Evaporation Chambers are good for this (note: a Purge Valve on the liquid-pipe between them is often necessary to prevent that pipe from breaking), but a phase change loop can also be made from pipes by using a Condensation Valve and a Purge Valve (set to 0kPa). By heating the colder liquid-side, or cooling the warmer gas-side, it's possible to keep the heating / cooling going.
The performance of these loops seems moderate at best, but they consume less energy than an Air Conditioner. The efficiency has not been measured.
Examples of how to take advantage of phase changes
1) Liquid tanks can be given a pressurant gas (like Oxygen) to prevent the liquid from evaporating. This will prevent any unwanted temperature changes from evaporation, and also makes it possible to remove every mol of the liquid from the tank.
2) on Europa, use an Active Vent to pressurize atmospheric Oxygen in a pipe with a least one Passive Liquid Drain. The high pressure will result in condensation which will heat the remaining gas in the pipe up to -111°C (from around -145°C). This isn't much, but could potentially be used to reduce the energy needed to heat up atmospheric Oxygen to room temperature.
3) on Mars, capture cold night-time atmospheric gas in a pipe with a small / moderate volume, preferably with a fast Powered Vent. Since only Pollutant and CO2 can phase-change into liquids below -8°C, both of these can be removed from the pipe via Passive liquid drains. The O2 and N2 however remains trapped inside. The condensation of CO2 will heat up the pipe (to -8°C), but if the temperature can be kept down the pipe will eventually contain a mix of just O2 and N2.
4) on Vulcan, water-steam near the night-time temperature (127°C) can be cooled with a phase change loop. Heated steam will accumulate on the gas-pipe side, and cooler liquid will accumulate on the liquid-pipe side. Expelling the heat from the hot gas-pipe will keep the cooling going. Radiators are not ideal since they will pick up a lot of heat during the day, a heat-exchanger on the other hand can be deactivated which makes it better (by using an Active Vent to provide it atmospheric gas only during the night, and a One-way Valve to let those gases escape on their own).
Footnotes
1) The Stationpedia says that all solids have 6.3kPa "Vapor Pressure". But a crude experiment showed that slowly pumping in a gas (CO2) into a freezing cold room (8 grids of O2 at -149°C) did not result in a significant change in pressure (when accounting for the small increase in temperature caused by adding a warmer gas). The added gas (CO2) did not show up on a Handheld Tablet (nor a Gas Sensor or Pipe Analyzer with its own separate pipe), the only way to spot the presence of the invisible "gas" was by the small amount of ice that formed inside the passive vent used to insert it into the room. After a while some ice (CO2) fell to the floor, without resulting in any changes of pressure or temperature. Doing the reverse, placing ice into a freezing cold room, will not sublimate any of that ice into gas. Based on this, it seems that un-frozen "gas" have no actual "vapor pressure", it will just invisibly accumulate until there is enough of it to form a lump of ice.
2) from https://www.reddit.com/r/Stationeers/comments/171842v/gas_pipe_stress_calculations/